Abstract
Introduction: Daratumumab is a CD38-targeting human IgGκ monoclonal antibody with a direct on-tumor and immunomodulatory mechanism of action. In combination with a proteasome inhibitor (bortezomib) or an immunomodulatory drug (lenalidomide or pomalidomide), daratumumab induces rapid, deep, and durable responses in patients with RRMM (Palumbo A, et al. N Engl J Med 2016. 375(8):754-766; Dimopoulos MA, et al. N Engl J Med 2016. 375(14):1319-1331; Chari A, et al. Blood 2017. Epub ahead of print). This analysis reports updated efficacy data within clinically relevant subgroups of DRd versus Rd in POLLUX.
Methods: Patients with ≥1 prior line of therapy were randomized (1:1) to receive Rd (lenalidomide: 25 mg PO on Days 1-21 of each 28-day cycle; dexamethasone: 40 mg PO weekly) with or without daratumumab (16 mg/kg IV weekly for Cycles 1 and 2, q2w for Cycles 3-6, then q4w until disease progression). Patients with creatinine clearance (CrCl) <30 mL/min were excluded from the study. The primary endpoint was progression free survival (PFS). The number of prior lines of therapy was determined by the site investigator and was based on IMWG consensus guidelines (Rajkumar SV, et al. Blood 2011. 117(18):4691-4695). Minimal residual disease (MRD) was assessed on bone marrow aspirates at the time of suspected complete response (CR) and 3 and 6 months after-suspected CR using the ClonoSEQTM next-generation sequencing (NGS) assay (V. 1.3; Adaptive Biotechnologies, Seattle, WA) at sensitivity thresholds of 10-4, 10-5, and 10-6. Patients were considered to be MRD positive if they had a MRD positive test result or had no MRD assessment. Cytogenetic abnormalities were detected by NGS. Patients with high-risk cytogenetics had ≥1 of t(4;14), t(14;16), or del17p abnormalities; standard-risk patients were confirmed to be negative for these abnormalities.
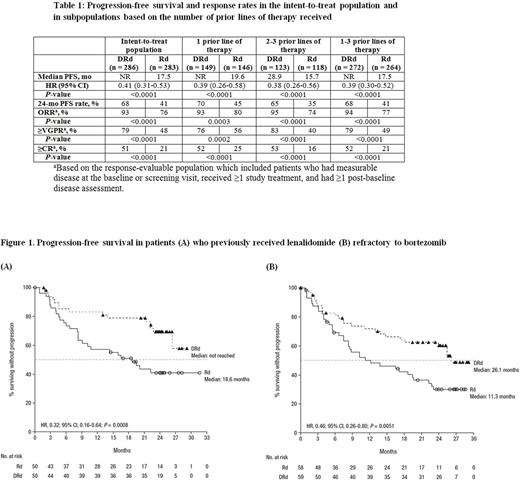
Results: A total of 569 patients were enrolled in POLLUX and baseline characteristics were balanced between treatment arms. At a median follow-up of 25.4 months, PFS was significantly prolonged with DRd versus Rd, regardless of the number of prior lines of therapy (1, 2-3, or 1-3; Table). The PFS benefit was maintained in patients who previously received lenalidomide (median: not reached [NR] vs 18.6 months; 24-month PFS rate: 69% vs 41%; HR, 0.32; 95% CI, 0.16-0.64; P= 0.0008; Figure 1A), were refractory to bortezomib (median: 26.1 vs 11.3 months; HR, 0.46; 95% CI, 0.26-0.80; P= 0.0051; Figure 1B), had moderately impaired renal function (>30-60 mL/min;median: NR vs 11.4 months; 24-month PFS rate: 63% vs 29%; HR, 0.36; 95% CI, 0.22-0.60; P <0.0001), or had received prior autologous stem cell transplant (ASCT; median: NR vs 19.9 months; 24-month PFS rate: 63% vs 45%; HR, 0.50; 95% CI, 0.36-0.69; P <0.0001).
Patients who received DRd achieved significantly higher overall response rates (ORRs) compared with Rd, including increased rates of very good partial response (VGPR) or better and CR or better, respectively, regardless of the number of prior lines of therapy (Table). ORRs were higher for DRd versus Rd among patients who previously received lenalidomide (84% vs 64%; P= 0.0233), were refractory to bortezomib (88% vs 68%; P= 0.0113), had impaired renal function (91% vs 68%; P= 0.0008), or had received prior ASCT (92% vs 79%; P=0.0004).
In terms of cytogenetic risk, the PFS benefit was maintained for DRd versus Rd in high (median 22.6 vs 10.2 months; HR, 0.53; 95% CI, 0.25-1.13; P= 0.0921) and standard risk (median NR vs 18.5 months; 24-month PFS rate: 74% vs 40%; HR, 0.30; 95% CI, 0.20-0.47; P <0.0001) patients. Patients who received DRd achieved higher ORRs compared with Rd in both the high-risk (85% vs 67%; P= 0.0435) and standard-risk (95% vs 82%; P= 0.0004) groups. A higher percentage of patients achieved MRD-negative status with DRd vs Rd, regardless of cytogenetic risk (high risk: 21% vs 0%, P= 0.0009; standard risk: 32% vs 12%, P= 0.0001).
Updated data will be presented at the meeting.
Conclusion: This updated analysis reveals that DRd provided clinical benefit to patients regardless of prior treatment history, cytogenetic risk or moderate renal impairment. This analysis demonstrates that DRd should be considered after lenalidomide-based therapies in patients with RRMM, including those refractory to bortezomib.
Moreau: Millennium: Consultancy, Honoraria; Bristol-Myers Squibb: Honoraria; Novartis: Consultancy, Honoraria; Celgene, Janssen, Takeda, Novartis, Amgen, Roche: Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Takeda: Honoraria; Amgen: Honoraria; Onyx Pharmaceutical: Consultancy, Honoraria. Oriol: Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: sponsored symposia, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: sponsored symposia, Speakers Bureau; Celgene: Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: sponsored symposia. Kaufman: Amgen, Roche, BMS, Seattle Genetics, Sutro Biopharma, Pharmacyclics: Consultancy; Amgen, Novartis: Research Funding. Sutherland: Janssen: Honoraria. Iida: Celgene: Honoraria, Research Funding; Bristol Mayer Squibb: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Ono: Honoraria, Research Funding; Chugai: Research Funding; Kyowa Hakko Kirin: Research Funding; Teijin: Research Funding; Astellas: Research Funding; Bayer: Research Funding; Eli Lilly: Research Funding; Sanofi: Research Funding; Daiichi-Sankyo: Research Funding. Prince: Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding. Cochrane: Novartis, Celgene, Bristol-Myers Squibb, Takeda, Amgen: Honoraria; Novartis, Janssen: Membership on an entity's Board of Directors or advisory committees. O'Rourke: Janssen: Employment. Wu: Janssen: Employment. Schecter: Janssen: Employment. Bahlis: Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal